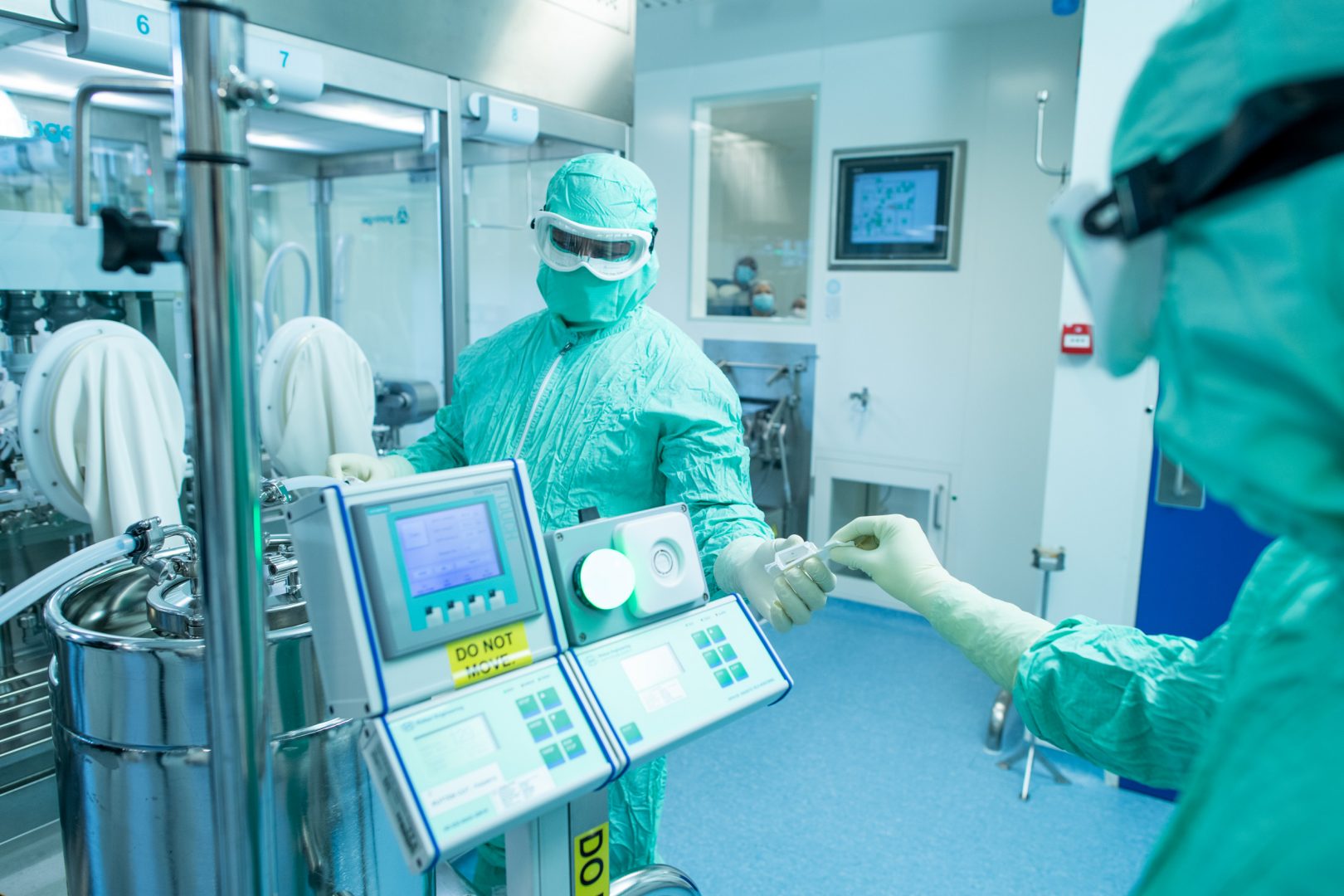
Gavi, the Vaccine Alliance, has approved the financing of the world’s first mass malaria immunisation programmes, using the ground-breaking RTS,S/AS01e vaccine. It is the first malaria vaccine to be proven safe and effective in a large Phase 3 clinical trial.
In August, MedAccess – a company founded by CDC in 2017 – agreed an innovative financing agreement to guarantee continued production of the vaccine antigen, while the World Health Organization and Gavi took decisions on the vaccine’s deployment.
Gavi’s board has now agreed to finance the life-saving vaccine’s rollout at scale for millions of children under-two in sub-Saharan Africa. This decision allows national health ministries to apply to Gavi to finance RTS,S procurement, so they can deliver it via existing national immunisation programmes.
Michael Anderson, CEO of MedAccess, said: “We stepped in with a production guarantee to keep manufacturing lines rolling. I am proud that this action will speed up vaccination against malaria for millions more children.
Gavi’s decision shows what can be achieved when science, public health and finance work in sync. The challenge now is to roll out the vaccine quickly alongside other proven malaria interventions, to protect people vulnerable to its devastating impact.”
Read more on this story on MedAccess’ website here.